Original
Hyaluronidase pre-treatment enhances collagen staining in sturgeon notochord
Pretratamiento con hialuronidasa mejora la tinción de colágeno en notocorda de esturión
Actual. Med. 2018; 103: (804): 72-75 DOI: 10.15568/am.2018.804.or03
Enviado: 12-06-2018
Revisado: 02-07-2018
Aceptado: 07-08-2018
ABSTRACT
Objective: The current study aimed to design a histological method to determine the presence and organization of the collagen network in sturgeon notochord.
Methods: Serial sections of sturgeon notochord (Acipenser naccarii) were used and assigned to two different experimental groups: Hyaluronidase pre-treatment (HP) in an alcohol acid solution (1% HCl in 70% alcohol solution) for 15 min and hyaluronidase solution in a 2 µg/ ml concentration (pre-heated at 37º C), and control (CTR) group, without pre-treatment. Then, the ECM was assessed by two histochemical methods: Picrosirius Red (PR) staining for 30 min with Sirius red (0.1% of Sirius red in saturated aqueous picric acid), for collagen bundle staining, and Alcian Blue (AB) staining for glycosaminoglycans detection.
Results: Samples analyzed in this study showed positive histochemical reaction for collagen fibers in both experimental groups. Referring to PR staining, the CTR group presented a larger and homogeneous reaction was observed in the entire samples, whereas HP group presented a more definite and intense pattern of collagen network. Also, this more intense signal in HP group matched with an increase of birefringence in polarized microscopy images of PR. However, HP group showed a lower intense and more heterogeneous signal when was compared with CTR group in AB staining.
Conclusion: Using a simple histological example, our study illustrates the capability of a hyaluronidase pre-treatment to enhance picrosirius red staining in sturgeon notochord trough light and polarized microscopy.
Keywords: Picrosirius-red, hyaluronidase, sturgeon, notochord, histochemical.
RESUMEN
Objetivo: El presente estudio tiene por objetivo diseñar un método histológico para determinar la presencia y organización de la red de colágeno en la notocorda del esturión.
Métodos: Secciones seriada de la notocorda de esturión (Acipenser naccarii) fueron utilizadas y se asignaron a dos grupos experimentales diferentes: pretratamiento de hialuronidasa (HP) en una solución de alcohol ácido (HCl al 1% en solución de alcohol al 70%) durante 15 minutos y posteriormente a una solución de hialuronidasa en una concentración de 2 μg / ml (precalentada a 37º C) y grupo de control (CTR), sin tratamiento previo. Luego, la ECM se evaluó mediante dos métodos histoquímicos: tinción Picrosirius Red (PR) durante 30 minutos con rojo Sirio (0,1% de rojo Sirio en una solución saturada de ácido pícrico), para la tinción de colágeno; y tinción con Alcian Blue (AB) para detección de glicosaminoglicanos.
Resultados: Las muestras analizadas en este estudio mostraron una reacción histoquímica positiva para las fibras de colágeno en ambos grupos experimentales. Con respecto a la tinción PR, el grupo CTR presentó una reacción mayor y más homogénea en toda la superficie de las muestras, mientras que el grupo HP presentó un patrón red de colágeno más definido e intenso. Además, esta señal más intensa en el grupo HP coincidió con un aumento de la birrefringencia en las imágenes de microscopía polarizada de PR. Sin embargo, el grupo HP mostró una señal menos intensa y más heterogénea cuando se comparó con el grupo CTR en la tinción AB.
Conclusión: Utilizando un ejemplo histológico simple, nuestro estudio ilustra la capacidad de un pretratamiento de hialuronidasa para mejorar la tinción de picrosirius en la notocorda del esturión a través de la luz y la microscopía polarizada.
Palabras clave: Picrosirius, hialuronidasa, esturión, notocorda, histoquímica.
Leer Artículo Completo
INTRODUCTION
Connective tissue is composed by cells and extracellular matrix (ECM), which is a complex network composed by proteoglycans (PG), glycoproteins and fibrillar proteins such as collagen and elastin (1). The glycosaminoglycans (GAGs) are the main components of PG and they are long unbranched and polar polysaccharides composed by repeating disaccharide unit (1). One of the repeating units consists of an amino sugar (N-acetylglucosamine or N-acetylgalactosamine) along with an uronic sugar (glucuronic acid or iduronic acid) (2). As a general rule GAGs are sulfated, such as heparin/heparan sulfate, chondroitin sulfate/dermatan sulfate between others, what makes them bind covalently with proteins forming glycoproteins. The only non-sulfated GAG is the hyaluronic acid which is a large macromolecule that cannot form covalent bounds with proteins by itself, and therefore binding proteins are needed to stablish the interaction with PG (1).
In relation to the fibers ECM, collagens are one of the most abundant proteins present in the ECM and are known to be rich in basic amino acids, concretely glycine, hydroxyproline and proline (3). These amino acids strongly interact with acidic dyes and collagen network are traditionally stained with Van Gieson and different trichrome methods with variable results (4). In this sense, the Picrosirius red method (PR) for collagen fibers, in which a solution composed by Sirius red F3BA dissolved in a satured picric acid solution, offer some advantages as compared to traditional Van Gieson and trichrome methods (5). Sirius red F3BA is an elongated anionic sulfonatedazo dye that colors collagen by reacting, via its sulfonic acid groups, with basic groups present in the collagen molecule. The elongated dye molecules are attached to the collagen fiber in such a way that their long axes are parallel. The parallel organization of the dye in the collagen surface results in an enhanced of the natural birefringence of these fibers making them selectively visible by polarizing microscopy (6-8).
Despite the many advantages offered by PR histochemical method some dense connective does not allow Sirius red to properly bind to the collagen molecules and it could be a problem in the characterization of new natural collagenous matrices that may be used in tissue engineering. The high density of the ECM in some species could affect colorant penetration and it could obstruct the reactions of dye molecules with the target binding sites. In order to solve this problem, previous studies have used pre-treatments before the stain procedure trying to facilitate the specific molecular groups’ interactions, such as papain or EDTA (7). The sturgeons emerged as potential source of natural biomaterials for biomedical applications (9). However, the tissue organization and molecular composition is poorly understood. The current study aimed to design a histological method to determine the presence and organization of the collagen network in sturgeon notochord.
MATERIALS & METHODS
This observational study was conducted on notochord tissue of one specimen of Acipenser naccarii which were provided by Caviar de Riofrío SL fish farm Riofrío, Granada, Spain. The live sturgeon (weight about 9 kg) was sacrificed and tissues were immediately frozen at -20 ºC until its processing. Tissues were defrosted at room temperature and the notochord was dissected and cut transversally in 40-50 mm sections.
Histological analysis
Samples of notochord were fixed for 24 h in 10% neutral buffered formalin solution in PBS (pH 7.4) at room temperature. They were washed in distilled water, dehydrated in graded alcohol and embedded in paraffin following a conventional protocol (10). All samples were cut in 5 μm thick sections for morphological and histochemical analyses.
Serial sections were used and assigned to two different experimental groups: Hyaluronidase pre-treatment (HP), and control (CTR) group, without pre-treatment.
In HP group, firstly samples sections were immerse in an alcohol acid solution (1% HCl in 70% alcohol solution) for 15 min to enhance the negative charge of GAG units. Secondly, hyaluronidase from bovine testes (Sigma-Aldrich, St Louis, USA) in a 2 µg/ ml concentration (pre-heated at 37º C) was applied to the sections that were incubated at 37ºC for 1 hour. Then, the ECM was assessed by two histochemical methods: PR staining for 30 min with Sirius red (0.1% of Sirius red in saturated aqueous picric acid), for collagen bundle staining (8, 11), and AB staining as previously described to determine reticular and collagen fibers and GAGs, respectively (8).
The sections were examined under a Nikon Eclipse 90i microscope, and images were captured with a Nikon Digital Camera DXM 1200c and NIS Elements software (Nikon, Tokyo, Japan) for light and polarized light microscopy.
RESULTS
Histology
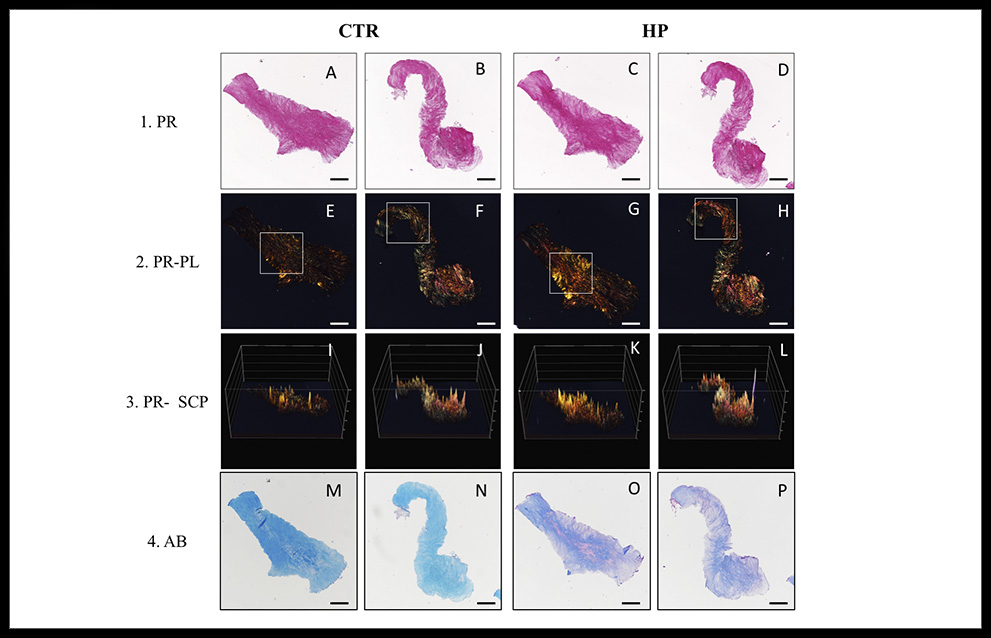
All samples analyzed in this study showed positive histochemical reaction for collagen fibers. In the CTR group a larger and homogeneous reaction was observed, but it reaction differed in comparison to the pattern, intensity and definition observed in the HP samples (Figure 1.1). Curiously, the HP allowed to identify well-defined collagen fibers which were intensely stained (Figure 1.C,D).
On the other hand, polarized light microscopy revealed the typical birefringence of the collagen fibers (red, yellow, orange and green) in all cases with some differences (Figure 1.2). Interestingly, the yellow and red birefringence was considerably enhanced in specific areas of the tissues sections which were subjected to HP (Figure 1.2). These findings were confirmed by using the surface plot analysis, in which the areas with higher intensity were easily identified (Figure 1.3).
Concerning Alcian blue staining (Figure 1.4), a homogeneous and intense signal was obtained in CTR group in the entire samples (Figure 1M, N). In the case of HP group the Alcian blue histochemical method was less intense with a heterogeneous pattern (Figure 1O,P) confirming the extraction of hyaluronic acid-based PGs.
Figure 1. Illustrative sections of sturgeon notochord (A–P). CTR: group control using conventional histochemical techniques; HP: Hyaluronidase pre-treatment group; PR: Picrosirius Red staining in light microscopy; PR-PL: Picrosirius Red staining under polarized light; PR-SCP: surface color plot of the PR-PL images; AB: Alcian Blue staining; Referring to PR staining, the CTR group presented a larger and homogeneous reaction was observed in the entire samples, whereas HP group presented a more definite and intense pattern of collagen network. Also, this more intense signal in HP group matched with an increase of birefringence in PR-PL. This fact is corroborated by PR-SCP. However, HP group showed a lower intense and more heterogeneous signal when was compared with CTR group in AB staining. Bar = 200 μm.
DISCUSSION
The characterization of ECM components has always been crucial to reveal tissue origin or development. Nowadays the search of new biological collagenous matrices as biomaterial to be used in tissue engineering is still a challenge. Collagenous tissues in no-studied species of vertebrates could present some troubles when conventional histological techniques are used.
The current study aimed to determine the collagen presence in sturgeon notochord and its fiber distribution on ECM trough a novel and modified PR staining method. Thus, a hyaluronidase pre-treatment was tested as enhancer of the dying molecular interactions with collagen fibers in the useful PR histological technique.
In relation to characteristic birefringence of collagen fibers in PR staining, many studies relied on this staining to identify collagen types according to their colors under polarized light until few years ago (6, 12, 13). Controversially, other authors reported that the polarized colors of PR staining depend only on the thickness of the collagen fibers, the density of their packing and spatial arrangement, not on the composition of the specific collagen type within collagen bundles (14-16). Also, Raed Lattouf et al. (2014) study illustrates the inability of PR staining to differentiate collagen types, since the absorbed amount of polarized light by this dye strictly depends on the orientation of the collagen bundles due to picrosirius red-stained reconstructed connective tissue sections (collagen lattice) were observed under polarized light before and after rotation of the microscope stage (17). In this study, polarized images of same sections of notochord tissue were tested, presenting a more intense signal of yellow, red or green birefringence in the marked areas of HP group that matched with specific dyed areas observed in light microscopy. This fact suggests that these more intense definite areas stained in HP group were collagen fibers and not an unspecific signal.
The term “hyaluronidase” was introduced to denote specifically the enzymes that degrade HA (18). Hyaluronidase has been used for numerous clinical applications, such as adjuvant therapy in cancer and to expedite the dispersion and absorption of drugs, as well as it has been used in ophthalmological procedures in combination with local anesthetics, reduction of dermatological aging, among others (19, 20). This is the first study that uses hyaluronidase enzyme as pre-treatment of a histological method such as PR or AB stainings, with the purpose of splitting the glucosaminidic bond between C1 of the N-acetylglucosamine section and C4 of a glucuronic acid in HA (21) producing density decrease and increasing the permeability of sturgeon notochord ECM. AB staining contains four tetramethylisithioronium groups that react binding to carboxyl and sulphate-ester groups at acid medium (pH 2.5 or 1) in carbohydrates molecules (3). Observing AB sections, the loss of expression observed in HP group suggests that the cleavage (at least partially) of HA (one of the major GAGs in ECM) was effective. This fact may decrease the viscosity and increase the permeability of ECM and produce the more intense dye on PR method together with a mildly loss of AB staining.
In this milieu, histochemical methods play a key role in the characterization of ECM in native and artificial tissues (22) and, in consequence, an adequate customization of these histochemical techniques should be carried out in accordance to the specimen to be studied, specially in those samples with high ECM density, as sturgeon notochord, in order to improve the accuracy of the obtained expression. In this sense, a HP could be used for a better detection of collagen fibers in such tissues.
Moreover, further research focused on deeper characterization of molecular composition by immunostaining should be carried out as a complement that could provide more information about molecular reactions taking place between tissues and dyes.
CONCLUSIONS
As we hypothesized, the large size and high presence of HA in the collagenous matrices could obstruct the interactions between molecules of dying and target sites producing not as much specific PR stain of collagen fibers. The hyaluronidase pre-treatment permits to degrade, at least partially, HA in sturgeon notochord and shows a more intense and define pattern of collagen network in PR histological in light and polarized microscopy. Finally, further studies are still required in others collagenous tissues to assess the potential use of hyaluronidase as a pre-treatment enhancer of PR staining for collagen fibers.
REFERENCES
- Gartner LP, Hiatt JL, Gartner LP. Color atlas and text of histology. 6th ed. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2014. xviii, 525 p. p.
- Varki A. Essentials of glycobiology. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1999. xvii, 653 p. p.
- Kiernan JA. Histological and histochemical methods : theory and practice. 3rd ed. Oxford ; Boston: Butterworth Heinemann; 1999. x, 502 p. p.
- Rich L, Whittaker P. Collagen and picrosirius red staining: a polarized light assessment of fibrillar hue and spatial distribution. Braz J Morphol Sci. 2005;22(2):97-104.
- Sweat F, Puchtler H, Rosenthal SI. Sirius Red F3ba as a Stain for Connective Tissue. Arch Pathol. 1964;78:69-72.
- Montes GS, Junqueira LC. The use of the Picrosirius-polarization method for the study of the biopathology of collagen. Mem Inst Oswaldo Cruz. 1991;86 Suppl 3:1-11.
- Junqueira LC, Bignolas G, Brentani RR. Picrosirius staining plus polarization microscopy, a specific method for collagen detection in tissue sections. Histochem J. 1979;11(4):447-55.
- Carriel VS, Aneiros-Fernandez J, Arias-Santiago S, Garzon IJ, Alaminos M, Campos A. A novel histochemical method for a simultaneous staining of melanin and collagen fibers. The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society. 2011;59(3):270-7.
- Wang L, Liang Q, Wang Z, Xu J, Liu Y, Ma H. Preparation and characterisation of type I and V collagens from the skin of Amur sturgeon (Acipenser schrenckii). Food Chem. 2014;148:410-4.
- Carriel V, Campos F, Aneiros-Fernandez J, Kiernan JA. Tissue Fixation and Processing for the Histological Identification of Lipids. Methods Mol Biol. 2017;1560:197-206.
- Oliveira AC, Garzon I, Ionescu AM, Carriel V, Cardona Jde L, Gonzalez-Andrades M, et al. Evaluation of small intestine grafts decellularization methods for corneal tissue engineering. PLoS One. 2013;8(6):e66538.
- Binnebosel M, Klink CD, Otto J, Conze J, Jansen PL, Anurov M, et al. Impact of mesh positioning on foreign body reaction and collagenous ingrowth in a rabbit model of open incisional hernia repair. Hernia. 2010;14(1):71-7.
- Cavallo JA, Roma AA, Jasielec MS, Ousley J, Creamer J, Pichert MD, et al. Remodeling characteristics and collagen distribution in synthetic mesh materials explanted from human subjects after abdominal wall reconstruction: an analysis of remodeling characteristics by patient risk factors and surgical site classifications. Surg Endosc. 2014;28(6):1852-65.
- Dayan D, Hiss Y, Hirshberg A, Bubis JJ, Wolman M. Are the polarization colors of picrosirius red-stained collagen determined only by the diameter of the fibers? Histochemistry. 1989;93(1):27-9.
- Pierard GE. Sirius red polarization method is useful to visualize the organization of connective tissues but not the molecular composition of their fibrous polymers. Matrix. 1989;9(1):68-71.
- Coleman R. Picrosirius red staining revisited. Acta Histochem. 2011;113(3):231-3.
- Lattouf R, Younes R, Lutomski D, Naaman N, Godeau G, Senni K, et al. Picrosirius red staining: a useful tool to appraise collagen networks in normal and pathological tissues. The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society. 2014;62(10):751-8.
- Hobby GL, Dawson MH, Meyer K, Chaffee E. The relationship between spreading factor and hyaluronidase. The Journal of experimental medicine. 1941;73(1):109.
- Wohlrab J, Finke R, Franke WG, Wohlrab A. Clinical trial for safety evaluation of hyaluronidase as diffusion enhancing adjuvant for infiltration analgesia of skin with lidocaine. Dermatol Surg. 2012;38(1):91-6.
- Khan N, Niazi ZR, Rehman F, Akhtar A, Khan MM, Khan S, et al. Hyaluronidases: A Therapeutic Enzyme. Protein Pept Lett. 2018.
- Rao V, Chi S, Woodward J. Reversing facial fillers: interactions between hyaluronidase and commercially available hyaluronic-acid based fillers. J Drugs Dermatol. 2014;13(9):1053-6.
- Martin-Piedra MA, Garzon I, Gomez-Sotelo A, Garcia-Abril E, Jaimes-Parra BD, Lopez-Cantarero M, et al. Generation and Evaluation of Novel Stromal Cell-Containing Tissue Engineered Artificial Stromas for the Surgical Repair of Abdominal Defects. Biotechnol J. 2017;12(12).
ARTICLE INFORMATION
Acknowledgments: The authors are grateful to Caviar de Riofrío SL Fish Farm (Spain) for providing the sturgeon specimen.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the authorship and publication of this article.
Corresponding Author: Óscar García. Dpto. de Histología de la Facultad de Medicina de Granada. Avda. de la Investigación, 11 · 18016 Granada. Correo electrónico: garciagarciaoscar2b@gmail.com